Posted on July 13, 2023 in ASRC News, Structural Biology Initiative
The discovery could help streamline new drug development.
More than 90% of clinical drug development efforts fail every year. A key factor driving the failure rate is that researchers currently have an imperfect method for determining early on whether and how small-molecule drug candidates bind to targeted disease-relevant proteins. A study by a multi-institutional team of researchers and recently published by the journal eLife could help finetune evaluation of small molecules, paving the way for new drug development.
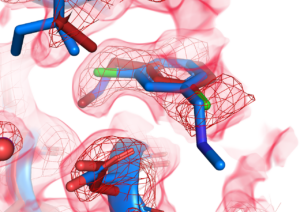
“The three-dimensional structure of a small molecule plays a key role in whether and how it will bind to a particular protein, so researchers spend a lot of time elucidating the shape and binding behavior of molecules that are potential drug candidates,” said the study’s principal investigator Daniel Keedy, whose lab is part of the ASRC’s Structural Biology Initiative. “Most often, structural analysis is done when the molecules are at cryogenic temperature, which is lower than -173 Celsius. We wanted to know whether the structure and binding behavior of small molecules would change at room temperature, which is much closer to real-life physiological conditions.”
To explore their question, the researchers designed X-ray crystallography experiments to track how small molecules that were previously screened at cryogenic temperatures would bind at room temperature to the protein PTP1B, which is known as the Achilles’ heel of diabetes and undruggable. The research team found that warmer temperatures had a significant effect on how the small molecules behaved. Some flipped their three-dimensional orientation by 180 degrees, some bound to entirely different sites on the protein, and still others bound to the same site but had different effects on the motions of the protein structure.
“These temperature-related variances show that the current method of visualizing how drug candidates interact with protein targets may not reveal the whole picture, and that we may need to screen small molecule drug candidates using multiple methods to fully understand their mechanisms,” Keedy said.
